Unveiling the hidden threat: nitrosamine impurities in medicines

Cancer-causing nitrosamines - also known as N-nitrosamines, N-nitroso compounds, or NOCs – first hit the headlines in 2018, when they were detected in batches of bestselling blood pressure drugs. In this article we recount the short but troubled history of pharmaceutical nitrosamine impurities, and global attempts to set acceptable intake limits for both small molecule nitrosamines and NDSRIs in drug products. We also discuss key challenges and advances in pharmaceutical nitrosamine testing, as well as the latest regulatory developments.
Becoming Public Enemy #1
Prior to threatening the supply of four in ten of the world’s medicines, carcinogenic nitrosamines had received comparatively little scientific attention. They were first detected in the 1870s, but it took until the mid-1950s for Magee and Barnes to link N-Nitrosodimethylamine (NDMA) with the development of malignant tumours in rats - while subsequent studies associated it with the deaths of farm animals fed on nitrite-treated herring meal.
Beginning in the 1970s, more rigorous research identified the presence of N-Nitrosamines in beverages, preserved food, personal care products, tobacco, and chlorinated or contaminated water – and also implicated them in the development of bladder, lung, liver, stomach and many other cancers.
However, it took the discovery of nitrosamines in pharmaceutical drugs to make regulators really sit up and take action - beginning in June 2018, when the US Food and Drug Administration (FDA) discovered NDMA in batches of the major Angiotensin Receptor Blocker valsartan. A month later, the European Medicines Agency (EMA) said NDMA and N-Nitrosodiethylamine (NDEA) had been detected in more sartans blood pressure drugs, while further testing later revealed unacceptable levels of NDMA in both the heartburn medicine ranitidine and the anti-diabetic metformin.
With numerous batch recalls beginning to threaten the global supply of these essential drugs, regulators on both sides of the Atlantic demanded that manufacturers carry out a comprehensive review of all human medicines for the possible presence of nitrosamines, and submit changes to their manufacturing processes where nitrosamines were detected above permitted levels. “After about three years or so, we thought we were pretty close to solving the whole problem,” said Astra Zeneca’s senior principal scientist in impurity management, Andrew Teasdale. However, the picture changed significantly with the emergence of a new class of nitrosamine impurities called nitrosamine drug-substance-related impurities (NDSRIs), which are intrinsically linked to the active pharmaceutical ingredients (APIs) of many drug products.
“All of a sudden [for maybe] 20%, 30%, even 40% of all existing drugs across major classes... there is a major risk of having to take these drugs off the market,” Teasdale told Chemistry World.
“We seemed to be heading towards a catastrophic loss of critical medicines.”

The multiple pathways to nitrosamine formation
Nitrosamine impurities can be formed in many different ways - the most common of which is an N-N bond formation process involving nitrites and vulnerable functional groups such as secondary or tertiary amines. Nitrite ions can readily transform into nitrosating agents such as N2O3 or NO+, especially in acidic conditions, and then react with amines to form nitrosamines.
Contamination can happen at virtually any stage of the life of a drug compound - from the purchase of ingredients to synthesis, and right up to storage of finished products. Nitrosating agents can be introduced throughout the production process via raw materials and the use of recycled solvents, reagents and catalysts. Chemical processes - such as quenching with a nitrosating agent in the presence of DMF (dimethylformamide) and the use of buffers containing tertiary or quaternary amines to stabilise APIs - have also been identified as key pathways for nitrosamine formation. Degradation can still occur once a drug product is finished and packed, through the use of unsuitable primary packaging, such as nitrocellulose blisters, or printing inks. Furthermore, nitrite impurities are found in most common drug excipients, at least in traces, and are therefore another potential contributor to the formation of nitrosamines.
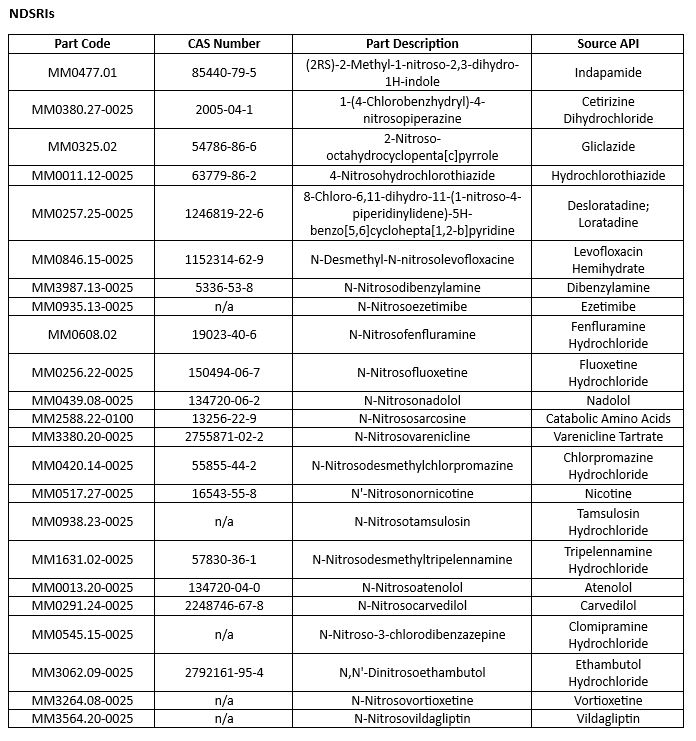
But most in danger of nitrosamine formation are the wide range of drug products whose API structures are comprised of secondary or tertiary amines, or tertiary ammonium salts. As Teasdale explains, “If the drug itself was a secondary amine, particularly if you’re using processes like wet granulation where you’ve got a fair amount of water present, you can dissolve the trace nitrite that’s commonly present in [other ingredients, and] you’ve got the chemistry conditions to form a nitrosamine.” These nitrosamine impurities – known as NDSRIs – are unique to individual APIs.
Alternative excipients, and other mitigation strategies
Because N-N=O bonds can occur due to many different precursors and conditions, preventing their formation has been described as “a multifaceted problem with no single solution.” However, a number of proven mitigation steps have emerged as our understanding of nitrosamines has grown since 2018. These include careful auditing of supply chains and testing of raw materials, as well as thorough consideration of where secondary and tertiary aliphatic and aromatic amines may occur during the production process – “including those present as part of the starting material, intermediate or final structure as well as those introduced as reagents, catalysts, solvents or as impurities.” Excipients containing the nitrosamine inhibitors ascorbic acid and alpha-tocopherol are another regulator-sanctioned response - since ascorbic acid in particular reacts with a number of nitrosating agents, and both compounds “bring the added benefit of acting as stabilizers in the finished drug product formulation without safety concerns.” Another potential approach is to use excipients with lower nitrite levels than those used previously. A study last year by Boetzel et al. found that crospovidone and magnesium stearate excipients contained significantly higher levels of nitrites than corn starch, lactose monohydrate and microcrystalline cellulose versions.
Challenges and changes in nitrosamine analysis
Since the panicked days of mid-2018, considerable progress has been made on understanding and limiting the threat of small molecule nitrosamine impurities in drug products. According to the EMA, the global response rate to regulators’ demands for risk evaluations of active substances and finished drugs stood at over 96% in September this year - with just 2.5% of total products assessed so far containing greater than permitted levels of nitrosamines. These Acceptable Intake levels (AIs, see Table 1) are necessarily strict, in line with International Council for Harmonisation guidance “that any known mutagenic carcinogen, such as nitroso compounds, should be controlled at or below levels such that there is a negligible human cancer risk.” However, demanding limits also present testing laboratories with challenges when quantitating volatile nitrosamines at low levels. As one case study puts it: “High sensitivity must be achieved to fulfil the regulation requirements, and selectivity is also critical to avoid false positive noncompliant results.” The volatility and small molecular size of many impurities necessitates careful handling in order to avoid recovery issues, while NDMA analysis presents “specific challenges due to the small size and high polarity of both the drug and the contaminant.”
Care is also needed when employing one of the most important analytical techniques for NDSRIs – namely electrospray ionisation mass spectrometry (ESI-MS). The presence of the nitroso group means that NDSRIs are particularly prone to in-source fragmentation within the ESI source - “namely the loss of 30 Da (which) corresponds to the detachment of the NO radical from the protonated nitrosamine compound.” This in turn significantly affects the observed mass spectra, making the correct identification and quantification of target analytes more difficult. There are, however, two main ways of minimising the likelihood of this type of fragmentation – decreasing the decluster potential voltage, and optimising the temperature of the ion source to preserve analyte integrity.
Getting to grips with NDSRIs
When the hazards posed by NDSRIs first became apparent, there was “little to no safety data available” on these more complex nitrosamines – which in turn made it difficult to set definitively safe limits based on acceptable daily intake. However, in July this year, the EMA and FDA updated their guidance on nitrosamine impurities with the introduction of the Carcinogenic Potency Categorisation Approach (CPCA) – “a science-based predictive solution to recommending AI limits for NDSRIs” for which no direct mutagenicity data exists.
According to the FDA, CPCA “assumes that the α-hydroxylation mechanism of metabolic activation is responsible for the mutagenic and highly potent carcinogenic response observed for many nitrosamines. Structural features that directly increase or decrease the favorability of the activation mechanism, or that increase the clearance of the nitrosamine by other biological pathways, will have a corresponding effect on carcinogenic potency. Therefore, a prediction of the mutagenic potential and carcinogenic potency of an NDSRI can be generated based on its structural features.”
The guidance adds that, once these structural studies have been carried out, NDSRIs may be placed in one of five potency categories based on AI limits ranging from 26.5 – 1500 ng/day – enabling manufacturers “to identify the appropriate potency category and associated recommended AI limits for NDSRIs in APIs and drug products, and to facilitate development of methods for confirmatory testing of impurity levels in drug batches.” With most drugs expected to fall into the least restricted Category 5, CPCA seems likely to remove many of the nitrosamine-related pressures on drug supply. “For a lot of our existing drugs, it makes a huge difference,” said Teasdale, welcoming the new approach.
The FDA recently issued another important update to CPCA by publishing a list of >260 NDSRIs and their potency categories, together with details of a new analytical requirement – an enhanced bacterial reverse mutagenicity assay, known as the Ames Test, which can be used to assess whether an NDSRI poses a mutagenic risk. Laboratories performing the enhanced assay – which requires two nitrosamine positive controls that are known to be mutagenic – must do so according to The Organisation for Economic Co-operation and Development’s Test Guideline No. 471.
LGC Standards – for all your nitrosamine testing needs
We provide a large portfolio of highly pure small molecule nitrosamine and NDSRI reference materials and research tools – helping to ensure that your pharmaceutical testing, research and analysis all comply with FDA and EMA regulations. Why not browse some selected products below, or see our full nitrosamines range?
|