First in class drug shows promise in preclinical neuropathic pain trials
A “ridiculous idea” inspired by ocean submarines has led to the discovery of a non-opioid, first-in-class drug candidate for neuropathic pain.
Scientists attached a ‘chemical anchor’ to the anaesthetic drug propofol – avoiding side-effects by preventing it from penetrating the brain, but allowing it to sink into peripheral nerve cell membranes and inhibit the activity behind nerve pain.
The novel drug candidate not only significantly reversed neuropathic pain signs in laboratory rats, but also had no addictive properties or adverse effects elsewhere in the body.
The researchers behind the designer molecule – from Weill Cornell Medicine and the Burke Neurological Institute – believe that more ‘anchor-tethered’ drugs like theirs can now be developed for “any number” of membrane targets.
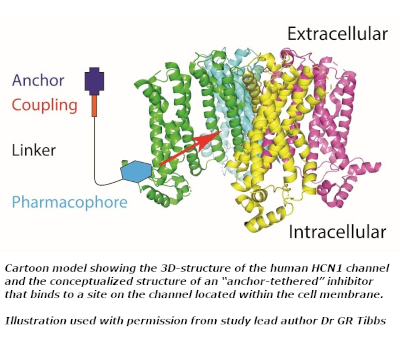
Previous studies, including work published by lead researchers Gareth Tibbs and Peter Goldstein, had established propofol as a promising candidate for inhibiting the activity of HCN ion channels that play a key role in nerve pain.
The difficulty with targeting HCNs, however, is that they are also widely expressed in heart and brain tissues, and so any novel drug would have to be designed to “spare the cardiac isoforms and… not cross the blood : brain barrier (BBB).”
Dr Tibbs’ group theorised that attaching a chemical anchor comprising a hydrophilic moiety and a carbon-based chain to an HCN1-targeted pharmacophore “could limit molecule penetration of cell membranes and the BBB while permitting or even enhancing pharmacophore access to its inhibitory site via the plasma membrane.”
The group tested the novel molecule via a combination of molecular dynamic simulation, electrophysiological recordings using Xenophus oocytes, plus an array of murine studies – and found that it performed precisely as expected. The orally-administered drug prompted a 4-fold slowing in ion-channel gating and thereby significantly reduced signs of hyperalgesia in the laboratory rats after both a single dose, and seven days of dosing. In addition, it proved non-addictive, and was also “poorly, if at all, penetrant to the brain.”

Developing a new class of non-opiate anti-hyperalgesic drugs to treat nerve pain successfully would be a significant achievement, given the huge personal and economic consequences of the condition. Drs Tibbs and Goldstein noted in their British Journal of Anaesthesia paper that around a fifth of chronic pain cases around the world are due to aberrant neuronal activity – not just blighting lives but also causing hundreds of billions of dollars each year of associated economic costs. Current treatments are, moreover, not just largely ineffective but also accompanied by “appreciable dose limiting side effects.”
Named BP4L-18:1:1 because of the 18 carbon atoms in its ‘anchor’, the new drug has been likened to a molecular ‘bathysphere’ – an early 20th-century submersible lowered into the ocean on a cable.
“In some ways it was a ridiculous idea,” said Dr Tibbs, “but what the current paper shows is that with the right group of collaborators, the ridiculous can become a reality.
“In so doing, we believe we have created a potential clinical anti-hyperalgesic, paving the way for developing an entire class of novel therapeutics that can be built to target any peripheral membrane-embedded protein of therapeutic interest.”
Dr Goldstein added: “These are encouraging results, and if further preclinical studies go well, we'll apply to begin an initial clinical trial.”
LGC Standards - for all your research chemical and drug discovery needs
As part of LGC Standards, TRC has more than 40 years’ experience working through some of the most complex synthetic pathways to deliver you high quality research chemicals. We have a uniquely large range - including APIs, bioactive molecules and SILS, as well as synthetic chemistry tools such as building blocks, linkers, spacers and coupling molecules.
LGC’s TRC range includes a significant number of pain and inflammation relevant chemicals: including agonists and inhibitors of opioid, prostanoid, cannabinoid, neurokinin receptors as well as ion channels such as HCN1-4.
Can’t find what you need? Our synthetic chemics are up to the challenge! Enquire at info.trc@lgcgroup.com
To support neurobiology in general, ATCC offer a large collection of in vitro tools, including NPC, Schwann cells and more than 70 brain lines.
|
